Let's imagine a few eukaryotic cells in a primitive pond. Floating freely, taking food with the apparent sole purpose of dividing and propagating their genetic information generation after generation. Now, let's suppose a period of food scarcity comes. The cells best adapted to starvation will be those that survive and can hold out until periods of abundance return. It's logical to think that these repeated situations over time will lead to the development of sophisticated mechanisms for adapting to starvation. Well, one of these mechanisms is autophagy, a process millions of years old that we only began to understand about 50 years ago.
Autophagy taught us to recycle and cope with hunger
Since we were little, we were taught the rule that plastic went in the yellow bin, cardboard in the blue bin, and glass in the green bin. This simple division makes recycling materials much easier so we can reuse them, right? Well, it turns out our cells already knew this, because they've been recycling since we were born.
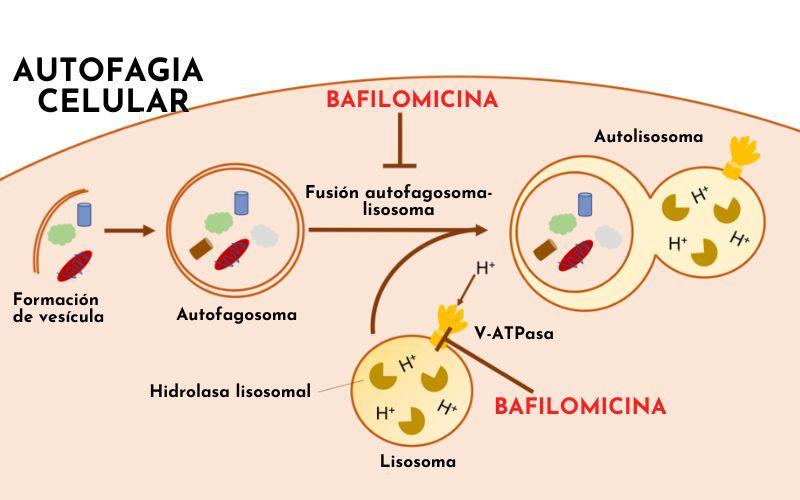
The cells in our body (and any eukaryotic cell in general, like the yeast that makes bread) are capable of wrapping and trapping organelles and proteins they no longer need within membranes and taking them to the organelle that is responsible for degrading and recycling (autophagy): the lysosome.
In this way, they are able to reuse "garbage" components in the most convenient way for them at any given time. When cells fast, they recycle many more molecules. Why? Because they need to adapt to the situation and survive, so they use everything they can reuse.
A tool for transformation
We all come from the zygote, which is the cell resulting from the union of sperm and egg. It's impressive to think how, from that single cell, an organism as complex as the human being can be generated. It's therefore inevitable that during development there are transformations of some cells into others, which is known as cellular differentiation.
Well, it has been detected that autophagy is necessary for some of these cellular transformations. And why? Well, mainly to eliminate everything the cell won't need in its new life.
An example would be the formation of red blood cells (the cells responsible for transporting oxygen in the blood). It has been observed that mice with mutants for genes involved in the autophagy process ended up developing anemia (a shortage of red blood cells).
And the most incredible thing of all is that the cell is able to distinguish what it wants to degrade through autophagy and what it doesn't. For example, in the case of the formation of retinal ganglion cells (which carry visual information to the brain), what is selectively degraded for cellular transformation are the mitochondria, which are responsible for obtaining energy.
Facing Viruses, Bacteria, and Diseases
Having all this machinery ready to wrap up trash, why not use it to degrade viruses and bacteria? It sounds like science fiction, but it isn't. Our cells have also learned to use this packaging and degradation process to defend themselves against infections.
But not all that glitters is gold. Some bacteria and viruses have evolved to the point where they can take advantage of the autophagy mechanism to go unnoticed and grow more easily.
Being such a basic and essential process for cells, it's no surprise that it's also implicated in a multitude of diseases, especially neurodegenerative diseases, but also inflammatory, autoimmune, and oncogenic diseases.
In most neurodegenerative diseases, there is an accumulation of some protein, which automatically leads to the belief that there is a failure in the protein degradation system. Many mutations have also been detected in genes related to autophagy, which are risk factors for these diseases.
But it doesn't only seem to be related to the ability to degrade accumulated proteins. Autophagy is also known to be important for the formation and elimination of synapses (neuronal connections) during development. One of the signs of autism is an excess of these neuronal synapses, and indeed, mice with autistic behavior (with the Tsc2+/- mutation) have been shown to improve if treated with autophagy inducers.
The main risk factor for these neurodegenerative diseases is old age. Again, autophagy appears to play a key role here. Scientists have managed to make flies, worms, and mice live longer by inducing this process. But it's one thing to do this in these animals, and quite another to safely transfer these results to humans and the clinic.
One process to control them all?
Anemia, neurodegenerative diseases, autism, aging… is autophagy the definitive cure for all these problems? Being such a basic process in the life of a cell, it's normal that it's affected in various diseases. For this reason, great care must be taken when modulating it. It wouldn't make sense to increase the formation of membranes that collect cellular waste if the process responsible for degrading it is obsolete, for example.
Ultimately, to be able to use autophagy to our advantage, it's essential to understand which part of the process is affected in each disease. And then decide whether it's wise to use one target or another.
In any case, we are undoubtedly looking at a potential therapeutic target. A multitude of companies have already launched a frantic race to find drugs that modulate autophagy safely and successfully in different clinical contexts.




:format(jpg)/f.elconfidencial.com%2Foriginal%2Fd39%2F916%2Fd2b%2Fd39916d2b2fb2980c9d84675c0c6f99c.jpg)